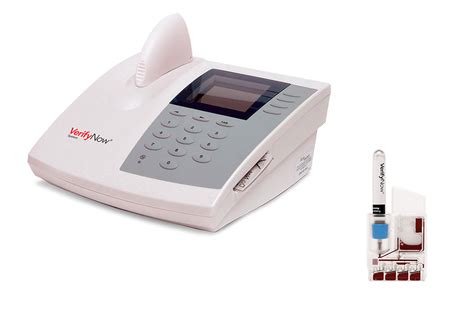
verifynow pru test package insert|verifynow platelet testing : private label VerifyNow PRUTest* Measures the level of platelet P2Y12-receptor blockade to help identify patient response to antiplatelet therapy, including clopidogrel (Plavix), prasugrel (Effient) and ticagrelor (Brilinta). web3521 Maclay Boulevard Tallahassee, FL 32312 850-431-BFIT(2348) 850-431-BFIT(2348) Directions
{plog:ftitle_list}
AirPlay. Celular ou Tablet. Baixe o aplicativo do Globoplay no seu aparelho pela loja de aplicativos Google Play (Google) ou App Store (Apple); Clique no ícone de usuário no .
cylindar head compression tester
The VerifyNow PRUTest measured changes in PRU after clopidogrel administration ranging from a minimum of 18 PRU to a maximum of 435 PRU with a mean change of 185 PRU.VerifyNow PRUTest* Measures the level of platelet P2Y12-receptor blockade to help identify patient response to antiplatelet therapy, including clopidogrel (Plavix), prasugrel (Effient) and ticagrelor (Brilinta).
Refer to VerifyNow IIb/IIIa package insert. This message appears if the entered baseline is outside the reference range. A baseline result is entered that is not within the 1) Verify that the baseline result entered was correct.VerifyNow PRUTest*. Measures the level of platelet P2Y12-receptor blockade to help identify patient response to antiplatelet therapy, including clopidogrel (Plavix), prasugrel (Effient) and ticagrelor (Brilinta). *Not available in all countries.The VerifyNow PRUTest is a whole blood test used in the laboratory or point-of-care setting to measure the level of platelet P2Y12 receptor blockade. Each test device contains lyophilized .Conditions that May Affect Test Results • Patient’s exposure to GP IIb/IIIa inhibitors within 48 hours of eptifibatide (Integrilin ®) or tirofiban (Aggrastat), or 14 days of abciximab (ReoPro ®). .
cylinder compression test head gasket
VerifyNow PRUTest Uses ADP and PGE1 to measure the degree of P2Y12 platelet-receptor blockade, identifying patient response to a P2Y12 inhibitor. VerifyNow Aspirin Test Utilizes an .VerifyNow PRUTest. Measures the level of platelet P2Y12-receptor blockade to help identify patient response to antiplatelet therapy, including clopidogrel (Plavix), prasugrel (Effient) and ticagrelor (Brilinta).The Verify Now system is an FDA approved test to measure and quantitate platelet responsiveness to a variety of antiplatelet medications including aspirin or P2Y12 inhibitor .VerifyNow Aspirin Test. Aids in the assessment of Aspirin's affect on platelets, allowing rapid, informed treatment decisions. VerifyNow PRUTest* Measures the level of platelet P2Y12-receptor blockade to help identify patient response to .
Test Code LAB5414 VerifyNow Clopidogrel (P2Y12) Screening Test Important Note. Previously listed as Clopidogrel (P2Y12) Screening Test. . Package insert: VerifyNow P2Y12, 14353.D. Accumetrics, Inc, 04/27/2009. . Reference interval from adults not taking aspirin is 169-356 PRU. Literature Reference: Prabhakaran S, Wells KR, Lee VH, Flaherty .VerifyNow Aspirin Test Aids in assessing how Aspirin is affecting platelets, allowing rapid, informed treatment decisions. VerifyNow PRUTest* Measures the level of platelet P2Y12-receptor blockade to help identify patient response to .
According to the VerifyNow package insert, . A study of pre-procedure platelet reactivity testing using VerifyNow found that a PRU value of <60 or >240 was the strongest independent predictor of all major peri-operative thromboembolic and haemorrhagic complications after PED . VerifyNow P2Y12 Platelet Reactivity Test, package insert.The VerifyNow package insert instructs the user to test blood samples within 4 hours after blood draw. Three stability studies are in progress to evaluate the stability of VerifyNow P2Y12 assay devices. The first study is being performed to determine the shelf life of VerifyNow P2Y12 assay devices when stored at recommended storage temperature.ASA resistance, aspirin resistance, clopidogrel, Effient, Efient, Plavix, Prasugrel, Ticlid, ticolpidine, Verify Now, VerifyNow PRU Components. Code Name; VERASG: VerifyNow Aspirin Assay Group VERASP: VerifyNow Aspirin Assay . Expected range for patient not on P2Y12 inhibitory drug is 182-335 PRU. This test is not to be used for therapeutic .VerifyNow P2Y12 Platelet Reactivity Test [Package Insert]. Accumetrics, San Diego, CA, 2013. has been cited by the following article: TITLE: Implications of the VerifyNow P2Y12 Assay on Patient Outcomes. AUTHORS: Denise A. Sutter, Gregory S. King, Marintha R. Short
VerifyNow System User Manual Page 9 14340.J 1 Introduction Before you begin to use the VerifyNow System, it is appropriate to review the purpose of the test. 1.1 Platelet Function Testing In general, platelet function testing measures the activity of platelets. Therapies that inhibit plateletVerifyNow, the most cited and utilized FDA-cleared platelet reactivity test, may help identify patients at risk of a major adverse cardiac event (MACE) due to poor drug response . VerifyNow will tell you whether or not the antiplatelet medication you prescribed is safe and effective for your patient It is an important tool among leaders .
TEST KIT, VERIFYNOW PRU (25TST/BX) Product Images Compare . Features. The VerifyNow PRUTest is a whole blood test used in the laboratory or point-of-care setting to measure the level of platelet P2Y12 receptor blockade Each test device contains lyophilized fibrinogen-coated beads, ADP, bovine serum albumin, PGE1, and buffer .
VerifyNow Aspirin Test Aids in assessing how Aspirin is affecting platelets, allowing rapid, informed treatment decisions. VerifyNow PRUTest Measures the level of platelet P2Y12-receptor blockade to help identify patient response to antiplatelet therapy, including clopidogrel (Plavix), prasugrel (Effient) and ticagrelor (Brilinta). To accomplish the goal of having a test with reduced non-specific aggregation, the VerifyNow PRUTest uses prostaglandin E 1 (PGE 1) in addition to ADP to make the test more sensitive and specific for the effects of ADP mediated by the P2Y 12 receptor. A PRU > 208 identifies patients with HPR, an established marker of increased thrombotic risk .Contact Accumetrics Technical Support at 1-800-643-1640 Option 2 if further service is required. References: 1. VerifyNow Assay WQC, Package Insert, PN 14349 2. VerifyNow PRUTest, Package Insert, PN 14438 3. VerifyNow System User Manual, PN 14439 14982.K VerifyNow PRUTest Procedure Template Page 9 of 9Please contact Phlebotomy Services for tube availability prior to ordering the VerifyNow Assay. Due to the special handling and time sensitivity, this test will ONLY be drawn on. INPATIENTS: Spectrum Health Medical Center-Grand Rapids . Higher PRU values are indicative of ineffective antiplatelet drug inhibition of platelet activity.
Test Code VerifyNow PRUTest Clopidogrel Response Test with VerifyNow Important Note. . 182 – 335 PRU Applies to patients prior to the treatment with platelet inhibitors. Reportable Units. PRU (P2Y12 Reaction Units) Critical . There are no data on patients with platelet counts of <100,000/mL or inherited platelet disorders. 12 In a prospective study (n = 147), the reference range for baseline platelet function by using P2Y12 assay 194–418 PRU . Platelet reactivity was assessed using the VerifyNow® point-of-care assays, with high platelet reactivity on clopidogrel defined as either VerifyNow P2Y12 >208 PRU or ≥230 PRU based on prior studies. VerifyNow .
The VerifyNow P2Y12 assay is a point-of-care test that is widely used to assess the efficacy of ADP-receptor antagonists and identify clopidogrel HTPR 4. It measures ADP-induced platelet activation in citrated whole blood samples and reports results in P2Y12 reaction units (PRU). . VerifyNow PRU and BASE results strongly correlated with .The VerifyNow platform is one of the most user friendly point-of-care platelet function test systems because it produces rapid results at the patient bedside. The purpose of the present paper is to give insight into the principal mechanisms of the VerifyNow system, to discuss its clinical utility for the monitoring of antiplatelet therapy and .
Recent trials have demonstrated that high levels of platelet reactivity, measured by the VerifyNow assay, are associated with an increase 12-month risk of ischemic events in patients who undergo stenting for acute coronary syndromes, with a cut-off value of platelet reactivity units (PRU) = 240, with hazard ratios up to 2,73 for nonfatal MI and .by the VerifyNow Instrument, and no PRU result is reported. Wet Quality Controls • L’utilisateur ne doit pas réparer l’instrument VerifyNow. Si des réparations sont nécessaires, il faut renvoyer les Wet Quality Controls (WQC) are available and supplied separately (with an accompanying package insert) forThe VerifyNow-P2Y12 is a rapid assay that test platelet activity over 3 min and uses of the combination of ADP and prostaglandin E1 (PGE1) to directly measure the effects of clopidogrel on the P2Y12 receptor. . (PRU). Results: Clopidogrel therapy resulted in a mean 64.0+/-25.3% PRU reduction. No participant reached PRU inhibition below 10% of .
10. VerifyNow PRUTest [package insert]. San Diego, CA; Accriva Diagnostics. 11. VerifyNow Aspirin Test [package insert]. San Diego, CA; Accriva Diagnostics. “Testing with VerifyNow supports ongoing expansion of the frontiers of endovascular aneurysm treatment by helping minimize risk of perioperative complications. ” Josser E. Delgado, MDRefer to the VerifyNow Aspirin package insert for information to be considered for patients receiving NSAIDS. The Aspirin Test may be used in patients treated with selective COX-2 inhibitors, e.g. celecoxib (Celebrex®). . (PRU). PRU reports the amount of ADP-mediated aggregation specific to the platelet P2Y12 receptor, and is calculated as a .
verifynow werfen na
The VerifyNow PRU Test is designed to measure P2Y12 receptor blockade. Results of the PRU Tests are reported as P2Y12 Reaction Units (PRU). PRU measures the extent of platelet aggregation in the presence of a P2Y12 inhibitor Lower PRU levels are associated with expected antiplatelet effect. <180 PRU – suggests P2Y12 inhibitor effectControl section of this package insert for additional information. • If drawing blood for a CBC at the same time as sample collection for VerifyNow Aspirin Test, fill the CBC tube last. • Do not freeze or refrigerate sample. • Collection of the blood sample should be performed with care to avoid hemolysis or contamination by tissue factors.Results could be affected by the following: GPIIb/IIIa inhibitors, low HCT, low PLT or inherited platelet disorders. Patients with HCT<20% or PLT<50 K/uL cannot be tested with the VerifyNow Assay. Suggested timing for testing patients on P2Y12 inhibitory drugs is dependent on dosage given. VerifyNow P2Y12 Suggested Sample Draw Time:
verifynow platelet testing
verifynow patient portal
11 de nov. de 2022 · Valorant Heart Crosshair Code: 0;P;c;6;o;0.1;m;1;0t;5;0l;3;0o;1;0a;0.7;0f;0;1t;1;1l;5;1o;0;1a;0.7;1m;0;1f;0. How to Use .
verifynow pru test package insert|verifynow platelet testing